A hypothesis by definition does not assume truth or outcome. By asserting the outcome based on limited evidence you have indeed made an unproven asummption instead of waiting for an actual outcome.
Here is a hypothesis couched in and to test on your terms: if a heterologous vaccination regimen is deployed, then a better outcome in terms of safety, efficacy and effectiveness compared to homologous vaccination may follow. Because this is prefaced with "if" and the conclusory phrase includes "may", the truth or outcome of the hypothesis is not assumed. It is posited as a hypothetical. This hypothesis is capable of being supported (or falsified) through observation. Here are the observations which support that hypothesis, and do not falsify it:
12 May 2021 - UK Com Cov Study Preliminary Report
18 May 2021 - first Spanish study
9 June 2021 - first German study
16 June 2021 - second German study
25 June 2021 - UK Com Cov Study Report
26 July 2021 - South Korea Study
15 October 2021 - US Study
26 October 2021 - Quebec and British Columbia Studies
5 December 2021 - second US study
6 December 2021 - The Lancet Paper on Com Cov
7 December 2021 - Report on UK Com Cov Study
How do the studies above constitute "limited evidence"? The answer is they are not limited evidence. They vary in terms of locations and sizes of populations studied, and the timing of the studies. They are far from "limited".
How do these studies falsify the hypothesis? They don't. If the hypothesis was false, the studies would contradict it. None of them do so.
My statement that az + moderna is not universally accepted as best option as some here are asserting is true. I never claimed that 3 doses of Pfizer is best, but mrna boosters are what is being recommended everywhere boosters are in play.
Which of the studies above support the proposition that "az + moderna is not universally accepted as the best option"? What studies do you have access to that the rest of us don't which contradict the findings in the studies listed above? The studies to which the rest of us have access suggest that overall (safety, infections and hospitalisations), AZ + Moderna delivered the best outcome. It is true that there's not much in it, and different locations delivered slightly different outcomes on infections and hospitalisations between each of the various combinations, but that combination edged out the others overall.
Again, I invite you to produce material which contradicts this. If you cannot produce it, it is open to draw the inference that you do not have such material.
In the US, mRNA vaccines AND the adenovirus vector vaccine (which AZ is also) there available (J&J) are being recommended by the US CDC:
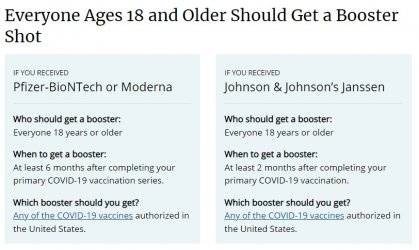
US CDC Booster Recommendations. The US CDC recommendation is very clear: "Which booster should you get?
Any of the Covid-19 vaccines authorized in the United States" (emphasis added).
Ive safely had 2 mrna doses and will have a 3rd mrna because its proven safe for me and effective. No one is recommending switching to AZ booster after 2 mrna doses.
As far I can ascertain, the reason that "no one" is recommending AZ third doses after two mRNA is because AstraZeneca has not been given marketing approval for that purpose. However, the US CDC is recommending that any US-authorised vaccine (which includes J&J, an adenovirus viral vector vaccine, which AZ is also) be used as a third dose: