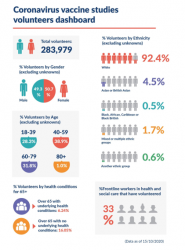
I’m delighted this newsletter is coming to almost 300,000 of you who have now signed up to the NHS Covid-19 Vaccine Research Registry, helping support our search for a safe and effective vaccine against Covid-19.
Last week I joined the thousands of you who have recently been called upon to take part in a vaccine study. My fellow study participants will be familiar by now with what is involved in a Covid-19 vaccine study, but I wanted to share my own experience with you.
Like all of you, I want to play my part in helping researchers quickly get the results they need to determine whether any of the vaccine candidates we have secured access to in the UK are effective against coronavirus. After I received the email inviting me to join a phase 3 study for a new vaccine developed by US biotech company Novavax, I completed the additional online screening forms and then received an evening call from the study physician. I kept my fingers crossed that this would work out as I couldn’t wait to get started.
Phase 3 studies involve many thousands of people, giving researchers insights into the effects of a vaccine on a much larger population than phase 1 and 2 studies. This helps us understand more about a potential vaccine’s safety and the dosage needed to generate protective immunity. What these studies are aiming to do is gather large amounts of information about how a vaccine behaves in a large number of people. This helps researchers determine if a vaccine is safe and effective, particularly for those most affected by Covid-19 such as the elderly, ethnic minorities and adults with serious underlying health conditions. 15,000 people from across the UK will take part in the Novavax study that I am part of over the next 13 months at a number of regional sites including Blackpool, Bradford, London, Stockport, Glasgow and Belfast.
I was extremely impressed with the ‘Rolls-Royce’ treatment I was promised all trial volunteers receive when I went along to the Royal Free London NHS Foundation Trust for my first injection. Having completed a pre-screening a few days before, which covered my health history and personal information to make sure I was eligible to take part in the study, I cycled over to the hospital for my personal appointment with the clinical study team. I was given a thorough examination to record the state of my health at the start of the study and was given a swab test to check for Covid-19 symptoms before I received my first injection of either the vaccine or placebo. I won’t know until the end of the study which one I received. I felt very well looked after at every stage, which was clearly explained to me and all my questions were answered. I was sent away with an electronic symptom diary installed on my phone, which I’ll use to track how I feel on a daily basis. I also have a number to call a physician any time day or night should I have any complications or concerns during the study.
With more studies due to start over the coming weeks, many more of you will receive an invitation to take part and I do hope you will join me and people like Faisal Ali, another Novavax study volunteer based in Bradford. Faisal is keeping a video diary detailing his journey so far, which is being documented by BBC Asian Network. You can hear what he has to say about his experience so far over on their Facebook page.
I will be returning to the study centre in a couple of weeks for my next ‘dose’, which is the protocol for this particular vaccine. I then have a number of follow-up appointments throughout the study, which will help researchers monitor my progress and collect that all-important information they need.